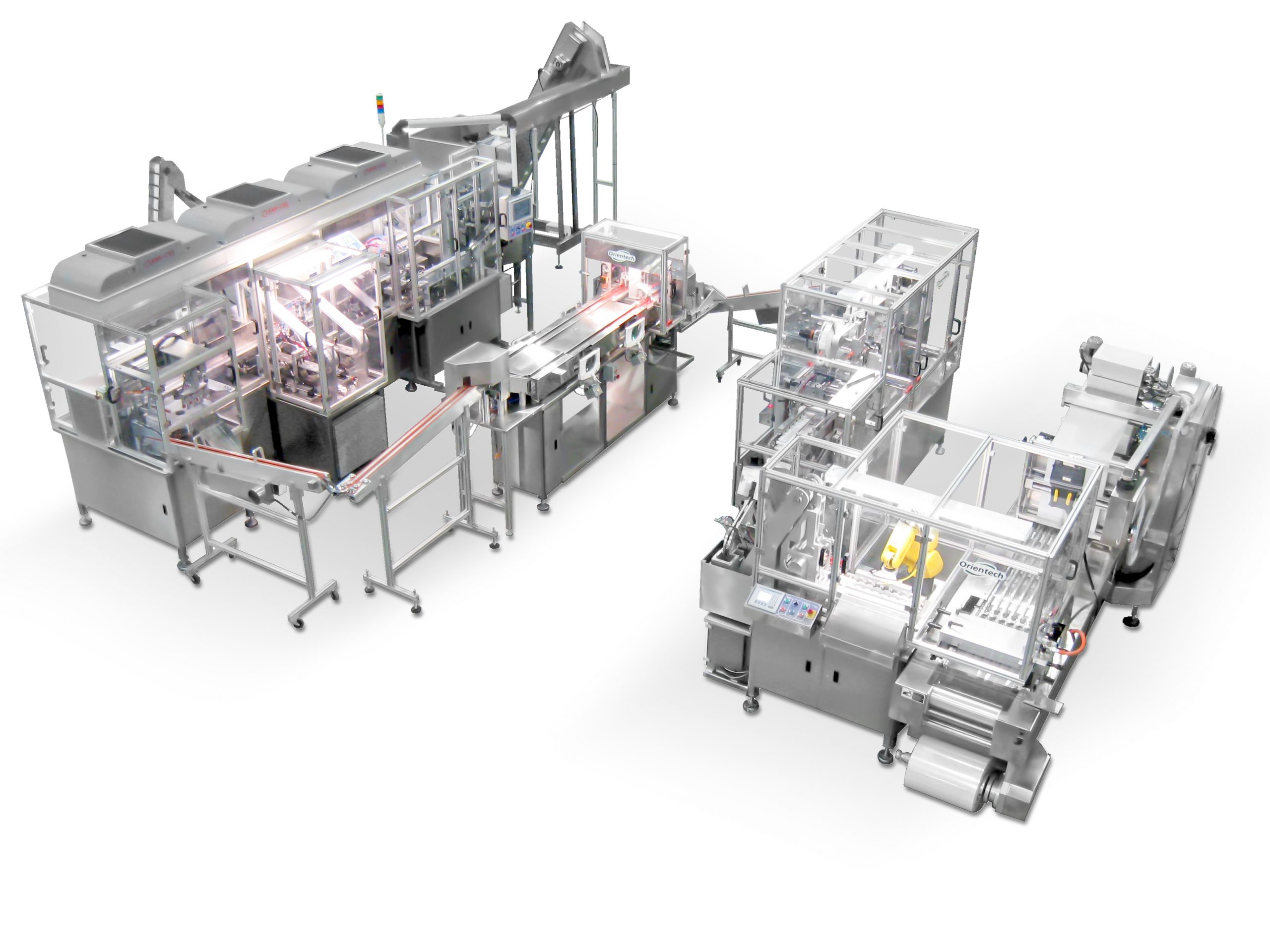
Our precise and flexible machines make it possible to produce and handle high-quality complex components frequently found in the pharmaceutical and medical sectors, such as oral syringe filling, vials, eye care products, test tubes, dip tubes, and dosing equipment.
Life Sciences
Pharmaceutical And Medical Devices
In-Depth Expertise In Designing Pharmaceutical Automation Needs
Orientech designs and manufactures automated and semi-automated systems that supply, direct, fill, and assemble components of different materials.
In the pharmaceutical sub-sectors, where regulation is an integral part of the production conditions, it is critical for a supplier to offer machines that strictly adhere to the many laws and regulations that apply to the different pharma sectors, including FDA requirements.
Due to its unparalleled knowledge, Orientech can adapt all the components of its assembly and feeding systems to the most rigorous standards for the production of pharmaceutical products.
Crucial processes, such as electro-polishing welding, are used when needed for our pharmaceutical projects, to ease sterilization requirements.
Orientech modifies system designs so that they can easily be integrated into an explosion-proof or white room, or for an easier sanitary wash.
Here are some of our realizations:
- Dropper assembly machine
- Child resistant cap assembly
- Semi-automatic syringe filling line
With our expertise and your input, we’ll implement the processes required to meet your needs and maximize your productivity.
Medical Devices
Medical device manufacturers cover a broad array of products in several medical specialties. In some cases, medical devices require high volume production assembly; in other cases, a high precision production assembly process is necessary. Also, packaging requirements for the end-users tend to differ.
Automation has become inevitable for repetitive and precision operations in the production of medical devices.
The integration of part or all of an assembly line depends on the level of automation required and the final scope of the medical device being produced. A detailed URS (User Requirement Specifications) allows us to determine commercial viability based on the necessary level of automation, the associated costs, and potential risks.
There are 1700 groups of devices classified between 16 medical specialties that are governed by the FDA in the United States.
Automation needs to be evaluated at a larger scale than just the equipment being integrated into a manufacturer’s facilities. Environment control is an essential factor in the assembly of medical devices.
Automation’s small footprint allows integrating smaller assembly lines into different classification areas. Guidelines are followed to meet the stringent classifications to meet governed approvals in the Medical Devices industry.
Count On Our Experience To Protect The Quality And Reputation Of Your Products
The opportunity to meet operational objectives is part of Orientech’s expertise, providing added value services by assembling medical devices having multi-part components that require particular in-line production needs.
Orientech’s cross-industry experience has brought depth to medical device automation while helping companies grow revenue by providing them with custom solutions. This enables companies to offer newly added services ranging from component feeding equipment, part orientation systems, multi-part component assembly lines, visual inspection cells, labelling, and stacking and packing components and final products. By understanding the overall “VSM” (Value Stream Mapping), these solutions improve different process areas. Efficiencies in run-rates, improved quality, and often lowered shipping costs, by stacking and packing, are a few examples of where operations are improved, or new packaging may run at an accustomed rate, with our automated and semi-automated solutions.
We take the time to understand your realities and put solutions in place to maximize every step of your product’s assembly process.
Here are some of our successful realizations:
- Mask and ear strap assembly system
- Catheter assembly machine
- Syringes assembly system
- Dropper assembly machine
Our work always considers the many standards that govern the pharmaceutical sector. Our assembly and feeding systems meet the strictest laws and regulations for hygiene and sterilization, and the components used for assembly lines are selected to respect your URS.
Our pharmaceutical clients entrust several of their automation needs to Orientech.

Check our dedicated solutions for the Life Sciences industry:
› Our Cap and Closure Lining Machine page
Orientech Solutions Used In Life Sciences Sector
We get involved early on in the design stage of products to help bring efficiencies while designing automated systems to assemble and package complex parts.
By using our services for the implementation of an automated assembly or feeding system, the production process of your products, which often seem simplistic, you will benefit from Orientech’s decades of cross-industry experience to further improve your existing process.
Medical device manufacturers have put their confidence in Orientech to handle the creation of their automated solutions for several devices such as oral syringe assemblies, saline syringe dosing, and capping, probing catheter assemblies, dropper assemblies to mention a few.
- Custom-built turnkey automated systems for multi-part assemblies
- Indexing and continuous assembly systems
- Cap lining/wadding equipment, including wide-mouth caps/closures
- Feeding and orienting systems (vibratory feeder bowls and centrifugal feeder bowls)
- Robotics: Orientech integrates FANUC robots and products, recognized for their speed, precision and durability
- Stacking and packing solutions
- Labelers
- Barcode readers
- Laser markers
- Small dose filling
- Vision inspection systems